DOI: 10.1039/C7RA13364B (Newspaper) RSC Adv., 2018, 8, 6001-6012
A molecular written report of modern oil paintings: investigating the part of dicarboxylic acids in the water sensitivity of modern oil paints†
Received 15th December 2017 , Accustomed 30th January 2018
First published on 6th Feb 2018
Abstruse
The 20th century has seen a significant development in artists' paint conception and applied science which is likely to relate to the new conservation challenges frequently presented by modern oil paintings, including unpredictable water- and solvent-sensitivity. This study examined the molecular causes and mechanisms behind these types of modern oil paint vulnerability. Research performed up to now has suggested a correlation between the occurrence of water sensitivity and the presence of relatively high amounts of extractable gratuitous dicarboxylic acids. To explore this farther, every bit well as the influence of paint formulation, a ready of model paint samples, produced in 2006 using commercial tube paints to which known amounts of additives were added, were analysed. The samples were tested for water sensitivity by aqueous swabbing and characterised using transmission Fourier Transform-Infra Crimson spectroscopy (FTIR) to determine the molecular composition of the main paint constituents, Loftier Operation Liquid Chromatography-Mass Spectrometry (HPLC-MS), to place the blazon(due south) of drying oils used every bit binders, and Gas Chromatography-Mass Spectrometry (GC-MS) using a recently developed analytical procedure that can discriminate and quantify costless fat and dicarboxylic acids, as well equally their corresponding metal soaps (carboxylates of fatty and dicarboxylic acids). The results indicated that the addition of modest amounts of additives can influence the h2o sensitivity of an oil pigment, likewise equally its molecular composition. Additionally the nature of the ionomeric/polymeric network appears to exist a significant determining gene in the evolution of h2o sensitivity.
1. Introduction
From the 18th century, artists' oil paint production evolved from small-scale production in studios, where paints were prepared from raw materials according to traditional and ofttimes well-protected recipes, to Colourmen, where paints were prepared in larger batches according to more than standardised procedures. In the nineteenth century, scientific and technological advancements enabled the mass production of creative person's oil pigment. The introduction of the collapsible paint tube in 1841, necessitated an increase in the complication of paint formulation in lodge to satisfy rheology and durability criteria for oil paints requiring a longer shelf life. 1 From the middle of the twentieth century, new synthetic polymers such equally acrylic and alkyd media became available, although 20 th century artists have continued to use paints based on drying oils: ii indeed, a recent survey at Tate found that more than than lxx% of their modern paintings contain oil media. 3 Modern artists' paints, including oil paints, can incorporate additions of metal salts, metal soaps, a multifariousness of dispersion agents, plasticizers, fillers and surfactants, among other materials, to influence specific properties such as rheology, stability, drying rate, and color. 1,4–8 Amidst these, metal soaps and/or free fatty acids (FFA) were added to oil-based paints as dispersing agents in order to improve the wetting properties of the pigments, and their ability to be homogeneously dispersed in the oil binder. 9–12
Some modern oil paintings are at present beginning to nowadays challenges for conservation: thirteen deterioration due to fabric composition and exposure to environment have led to surface changes, and in some cases, the migration and aggregation of chemical species occurs, leading to a range of issues such as the formation of vulnerable 'medium skins' on paint surfaces, efflorescence, protrusions, colour change and paint delamination, every bit well equally h2o- and solvent-sensitivity of pigment surfaces.
The sensitivity of unvarnished modernistic oil paintings to standard surface cleaning methods such equally swabbing with water or saliva has been reported past conservators working both in museums and individual collections. 9 In the last ten years this trouble has been the focus of interdisciplinary research involving conservators and scientists. 3,9,13,fourteen Research consortia such as the Mod Oil Enquiry Consortium (MORC)‡ and the European Articulation Program Initiative (JPI) Heritage Plus funded project on the Cleaning of Mod Oil Paints (CMOP), reflect the attention that this issue is currently receiving. 14
Water sensitivity has at present been identified in model oil pigment samples prepared from raw materials 13,15,sixteen {Storm, 2010 #16}, in samples taken from batches of manufactured paint, 14 and in numerous paintings. 16–18 Sensitivity may be limited to certain colours and passages, may affect the whole surface of a painting, may be specific to some paint brands or lines, and may affect specific pigments across several brands. 15,eighteen
Ane fundamental area of investigation involves the exploration of how paint formulations and additives may influence paint ageing and degradation processes. 3 The proven causes of sensitivity thus far identified include the germination of magnesium sulphate heptahydrate (epsomite) on some paint surfaces, as a result of the presence of magnesium carbonate in paint formulations, which tin can react with sulphur dioxide from the temper. 13–15,17 The presence of under-bound or lean paints tin as well contribute to sensitivity, which is may derive from artists' technique. three,nine Nonetheless, water sensitivity has also been observed in many well-bound (or 'fat') paints.
A related study identified a strong relationship between h2o sensitivity and pigment type: paints formulated with zinc oxide and/or lead were consistently non-h2o sensitive. 14,xix The study also demonstrated that in full general, water sensitive paints were not associated with a higher degree of oxidation compared with non-sensitive paints, although some highly oxidised paints (mostly containing Fe and Mn pigments) were often noted as water sensitive. 14 In general, irrespective of the overall degree of oxidation of the paint, water extracted a relatively higher corporeality of complimentary dicarboxylic acids from water-paints than from non-water sensitive paints. xiv Dicarboxylic acids, which are the products of oxidation of a drying oil, 20 exhibit a certain caste of h2o solubility: 2400 mg L −one (at twenty °C) for azelaic acid, versus 0.04 mg L −1 (at 25 °C) for palmitic acrid. This atomic number 82 to the hypothesis that a sample rich in relatively high amounts of free – unbound and non-saponified – dicarboxylic acids, may be water sensitive during cleaning, via swelling and weakening the pigment structure. On the other hand, where dicarboxylic acids are present as metallic soaps (as opposed to in free form), they are more probable to form a relatively stable, 3-dimensional metal coordinated network, due to their chain building power. 21
In this written report, these hypotheses were investigated further by exploring the relationship between water sensitivity and the composition and proportions of gratuitous and saponified dicarboxylic acids. A set of model cobalt bluish and raw sienna pigment samples exhibiting varying degrees of water sensitivity were characterised using transmission infrared spectroscopy (FTIR), liquid chromatography mass spectrometry (LC-MS) and gas chromatography mass spectrometry (GC-MS), the latter using a recently adult belittling process that can discriminate and quantitate free fat and dicarboxylic acids, and their corresponding metal soaps. 22
2. Materials and methods
2.i. Samples
Model paint samples vest to a collection prepared in 2006 at holland Constitute for Cultural Heritage (ICN) in 2006 using Winsor & Newton Artists' Oil Colours (WN) and Talens Rembrandt Oil Colours (TA), with cobalt blueish (CB) and raw sienna (RS) as pigments. Aliquots of paint were applied unadulterated or with add-on of one additive on Melinex® supports according to a standardised process, the details of which take been reported elsewhere. 11,18 The model paint samples were lite aged under indoor conditions at loftier lux§, and later on stored indoor in room ambient conditions until 2012, when they were dismounted and kept in darkness inside drawers. 23
The model paint samples used in this study are listed in Table 1 and additives used are 2% aluminium stearate (AS), 2% zinc stearate (ZS), two% free fatty acid (FA)–heptadecanoic acrid (margaric acrid). It is noted that the samples selected for this report did not contain detectable amounts of epsomite. 23 At low magnification, the surface of samples appeared to be well bound. 23 Every bit a result, water sensitivity of these samples could not be ascribed to the presence of soluble salts or under-bound paints.
Table 1 Composition of model paint samples as obtained from the manufacturer and elemental assay
| Manufacturer/serial | Brand colour (and number)/pigment used¶ | Pigment chemical limerick or formula | Elemental composition of unadulterated model paint samples a | Additives added during preparation of model pigment samples in 2006 (ref. xviii) | Model paint acronyms |
| Results obtained via SEM-EDX analysis of the unadulterated model paint samples. 23 |
| Winsor & Newton/Artists' Oil Colour | Cobalt bluish deep (180)/PB74 | Co–Zn silicate (Co, Zn) 2 SiO 4 | Co, Zn, si, O, Mg, C, Ba | None | WNCB |
| 2% margaric acid | WNCBFA |
| 2% Zn stearate | WNCBZS |
| 2% Al stearate | WNCBAS |
| Talens/Rembrandt Oil Colour | Cobalt blue (513)/PB28 | Cobalt aluminate (blue Spinel) (CoAl 2 O iv ) | Co, Al, O, Ca, C, Zn, Mg | None | TACB |
| 2% margaric acid | TACBFA |
| 2% Zn stearate | TACBZS |
| 2% Al stearate | TACBAS |
| Winsor & Newton/Artists' Oil Color | Raw sienna (552)/PY42, PY43 | Natural iron oxide, (PY43: Iron ii O 3 ·H ii O with impurities) constructed iron oxide (PY42: Atomic number 26 2 O 3 ·H 2 O) | Fe, O, Ca, C, 1000, Al, Si (Zn) | None | WNRS |
| ii% margaric acrid | WNRSFA |
| ii% Zn stearate | WNRSZS |
| two% Al stearate | WNRSAS |
Information technology is noted that the pigments used in the cobalt clue paints of Winsor and Newton and Talens are different. Pigment PB74 Co–Zn silicate is used in the Winsor and Newton cobalt blueish deep paint, while pigment PB28, cobalt aluminate is used in the Talens cobalt blueish paint.
2.2. Water sensitivity tests
The examination used to constitute the sensitivity of the paint surfaces to h2o was based on a semi-standardised method, used in previous studies, involving the rolled application of dampened cotton wool swabs to the pigment surface. 18,24 The swab scroll tests were performed twice, and evaluation of sensitivity was based on the average number of swabs rolls that could be applied to the paint surface until pigment particles were picked up onto the swab. Sensitivity criteria used are reported in Tabular array 2.
Table 2 Sensitivity criteria used to decide sensitivity of paint to swab rolling using deionised h2o
| Water sensitivity criteria | Nr swab rolls necessary to remove the pigment | Numerical indicator |
| Not sensitive | ≥31 | 1 |
| Moderately sensitive | 21–30 | 2 |
| Sensitive | 11–twenty | iii |
| Very sensitive | ≤x | 4 |
The paints used in this study were selected from a larger batch based on differences in h2o sensitivity behaviours. The selected paints range from very sensitive to not-sensitive, and include samples whose water sensitivity characteristics had changed since earlier tests were carried out. xi,23 .
2.3. GC-MS
Fragments of the model paint samples (600–800 μg) were subjected to a double derivatisation process in guild to separately analyse and quantify both free fatty acids and carboxylates of fatty and dicarboxylic acid (not leap to the polymeric network, nor to glycerides). 22 || Analyses were performed in triplicates for each model pigment, after crushing pigment fragments in an agate mortar to ensure sample homogeneity. Samples were augmented with a tridecanoic acid solution (5 μL, 125.eight ppm in isooctane) as an internal standard and so subjected to the first derivatization for the GC-MS assay by calculation derivatising amanuensis hexamethyldisilazane (HMDS, 20 μL, Sigma-Aldrich) and iso-octane solvent (50 μL, Sigma-Aldrich), and heated to sixty °C for thirty min. A 2nd internal standard, hexadecane, (97.23 ppm in isooctane) was also added before injection. An aliquot (ii μL) of the supernatant solution was injected into the GC-MS. The residual solution was then dried nether a nitrogen flow, and afterwards derivatised with the second derivatising agent, bis trimethylsilyltrifluoroacetamide (BSTFA, 20 μL, Sigma-Aldrich) in iso-octane (50 μL), heated at 78 °C for 80 minutes. An aliquot (two μL) of this solution was so injected into the GC-MS. Quantitation of lauric, suberic, azelaic, myristic, sebacic, palmitic, oleic and stearic acids, was performed using calibration curves built using standard solutions containing a mixture of the analytes in acetone (Sigma-Aldrich) in the range of ane–100 μg g −1 .
Analyses were performed with a GC-MS instrumentation consisting of an Agilent Technologies 6890N gas Chromatograph coupled with a 5973 mass selective detector single-quadrupole mass spectrometer. Samples were injected in splitless fashion at 280 °C and gas chromatography (GC) separation was performed on a fused silica capillary column HP-5MS (J&W Scientific, Agilent Technologies, stationary phase five% diphenyl 95% dimethyl-polysiloxane, 30 m length, 0.25 mm i.d., 0.25 μm film thickness). Chromatographic conditions were: initial temperature fourscore °C, two min isothermal, xx °C min −1 up to 280 °C, 10 min isothermal. MS parameters: electron touch ionization (EI, 70 eV) in positive mode; ion source temperature 230 °C; scan range 50–700 one thousand/z; interface temperature 280 °C. Analyses were performed both in Selected Ion Monitoring (SIM) and Total Ion Current (TIC) modes.
two.4. FTIR
The bulk of the model paint samples were analysed using transmission FTIR spectroscopic analysis, using a Thermo scientific Nicolet iN10 MX microscope with a single diamond cell, equipped with an MCT-A/CdTe detector. 64 scans were collected at a resolution of 4 cm −1 across a wavenumber range of 4000 to 675 cm −1 . Paint fragments were applied to a single diamond jail cell and rolled flat using a steel roller. Data were processed using Omnic eight software.
iii. Results and discussion
iii.ane. Water sensitivity tests
The unadulterated WNCB pigment was very sensitive to water, but the addition of 2% margaric acid or 2% aluminium stearate or 2% zinc stearate to these paints mostly acquired a slight decrease in sensitivity. The TACB (cobalt blue) paints were plant to exist only moderately sensitive to water. The event of the addition of Al and Zn stearates on the Talens cobalt blueish paint resulted in a decrease of the sensitivity to h2o swabbing. However, the addition of 2% margaric acid increased the sensitivity to water when compared to the same paint without additives. All of the WNRS (raw sienna) series were not sensitive to swabbing with deionised h2o, and in this case, the presence of the additives did non result in significant changes in water sensitivity. The results of the water sensitivity tests are summarised in Table iii.
Table 3 Results of water sensitivity tests to water
| Sample | Sensitivity to h2o |
| WNCB | 4 |
| WNCBFA | 3 |
| WNCBAS | 3 |
| WNCBZS | 3 |
| TACB | 2 |
| TACBFA | three |
| TACBAS | 1 |
| TACBZS | 1 |
| WNRS | 1 |
| WNRSFA | 1 |
| WNRSAS | 1 |
| WNRSZS | 1 |
3.2. FTIR
FTIR was used to characterise the molecular composition of the selected model paint samples and to gain data on the degree of hydrolysis of the paint and formation of metallic carboxylates. The spectra of the unadulterated samples are presented in Fig. 1–3.
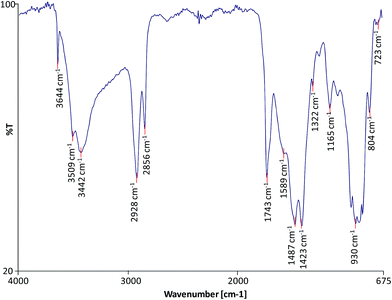 |
| | Fig. 1 FTIR spectrum of sample WNCB. | |
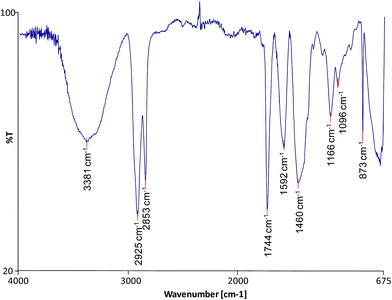 |
| | Fig. ii FTIR spectrum of sample TACB. | |
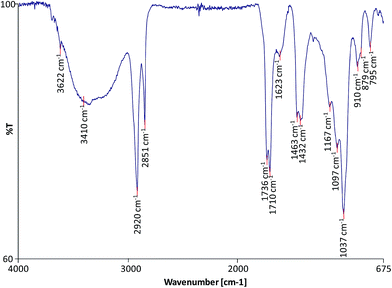 |
| | Fig. iii FTIR spectrum of sample WNRS. | |
All the spectra show the characteristic bands of an oil binder: the wide band centered at ca. 3440 cm −one is assigned to the stretching of alcohol and hydroperoxide bonds, the band at ca. 1740 cm −1 is assigned to the ester stretching, and the bands respective to the CH stretching are ca. 2928 and ca. 2860 cm −1 . The FTIR spectrum of sample WNRS (Fig. 3) shows likewise the presence of a band at 1710 cm −1 respective to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration related to the formation of free fat acids as a result of the hydrolysis of triglycerides, and, to a sure extent, oxidation with germination of dicarboxylic acids. This is in understanding with the results obtained for other paint reconstructions consisting of linseed oil and Fe based pigments (reddish ochre, Prussian blue and red bole) 25 confirming the observation that Fe-based pigments promote the hydrolysis of triglycerides. 26 Complimentary fatty acids could not exist distinguished in WNCB and TACB (Fig. 1 and 2), however this has been observed in other paints containing Co and Zn containing-pigments (zinc white and cobalt light-green). 14,25,27 A separate carbonyl band with absorptions at ca.1740 cm −1 and ca. 1710 cm −1 has been associated with h2o sensitive oil paints in a related study. 14,27
O stretching vibration related to the formation of free fat acids as a result of the hydrolysis of triglycerides, and, to a sure extent, oxidation with germination of dicarboxylic acids. This is in understanding with the results obtained for other paint reconstructions consisting of linseed oil and Fe based pigments (reddish ochre, Prussian blue and red bole) 25 confirming the observation that Fe-based pigments promote the hydrolysis of triglycerides. 26 Complimentary fatty acids could not exist distinguished in WNCB and TACB (Fig. 1 and 2), however this has been observed in other paints containing Co and Zn containing-pigments (zinc white and cobalt light-green). 14,25,27 A separate carbonyl band with absorptions at ca.1740 cm −1 and ca. 1710 cm −1 has been associated with h2o sensitive oil paints in a related study. 14,27
The spectra of both WNCB and TACB (Fig. one and two) showed the presence of a broad band centred at ca. 1590 cm −1 . Baggy Zn and Pb soaps are characterised past a broad band at ca. 1590 cm −1 (ref. 28) and ca. 1580 cm −1 (ref. 29) respectively, which are absorptions shifted ∼45 cm −1 toward college wavenumbers with respect to the absorptions of their corresponding crystalline Zn and Pb soaps at ca. 1540 cm −1 . 30,31 Assuming that the baggy metal lather ring for Co stearates is associated with a like shift in wavenumber with respect to its crystalline course, expected at ca. 1540 cm −one , 32 then the broad band at ca. 1590 cm −1 (Fig. ane and 2) may tentatively be ascribed to amorphous carboxylates of Zn and/or Co. The presence of the sharp band in the spectrum of WNCB (Fig. 1) at ca. 3650 cm −1 , together with the bands at ca. 3509 and ca. 3442, ca. 1487 and ca. 1423 cm −1 related to the CO three ii− vibration, and the sharp band at ca. 803 cm −i , correspond to hydromagnesite (a form of magnesium carbonate) known to be used in Winsor and Newton oil paints. 33 The bands at ca. 1430 and ca. 873 cm −one in the TACB spectrum are related to the presence of calcium carbonate (CaCO 3 ). This is in understanding with the presence of Mg and Ca in the elemental composition of these paint layers (Table 1). The use of the Co–Zn silicate–silicate pigment (PB74) in WNCB, (Tabular array 1), is farther confirmed by the broad band centered at ca. 930 cm −i and that at ca. 723 cm −i (Fig. one).
Sample WNRS shows the characteristic bands of raw sienna: ca. 3687, ca. 3621, ca. 1623, ca. 1037, ca. 910, 887 and 795 cm −1 ; related to the presence of kaolinite in the naturally sourced pigment (Fig. 3). Despite the presence of Ba in the WNCB, and Zn and Mg in TACB and traces of Zn in WNRS (Tabular array 1), 23 their molecular composition could not exist ascertained from the FTIR spectra. This is due to the fact that their diagnostic bands might be masked by other more than abundant bands, and/or these compounds might be nowadays in amounts below the detection limit, or beyond the conquering moving ridge range (oxides, sulphides, etc.). Ba is probable to originate from barium sulphate – a mutual paint extender, Mg to magnesium carbonate and Zn may originate from added stearates or ZnO, unremarkably added to pigment formulations. Added Zn stearates would exist in their crystalline grade and would thus prove a abrupt band at ca. 1536 cm −ane , 25 which is not visible in the spectrum of sample TACB. The sharp band at ca. 1321 cm −ane in the spectrum of sample WNCB (Fig. i) may be due to the presence of oxalates or a C–O assimilation from the oil medium. No other bands were present that would help to ostend the identification of the oxalate type. Mg, Zn and Co oxalates accept sharp bands in the range 1320–1325 cm −1 , which were not detected in these samples. 34,35
three.iii. GC-MS
Loftier-Operation Liquid Chromatography coupled to Electrospray Ionisation and Quadrupole Fourth dimension-of-Flying Mass Spectrometry (HPLC-ESI-Q-ToF) was used for triglyceride profiling 36–41 to identify the drying oil(s) used equally paint binders, the results of which are discussed in the electronic ESI.† In summary, all of the samples contained a mixture of drying and semi-drying oils: TACB contained safflower oil, WNCB a mixture of linseed oil and safflower oil, and WNRS a mixture of linseed oil and safflower. Castor wax is present in the WNCB pigment, and small amounts of triglycerides containing odd numbered fatty acids appear to be nowadays in the WNRS pigment. One previous report including the assay of Winsor and Newton oil paint model paints indicated that water sensitivity (inside the range of paints analysed) does not appear to relate to the type of oil, nor to the presence of brush wax, which does not appear to be consistently associated to water-sensitive paints. fourteen,42 Castor wax has been previously identified in commercial paints, likely as a stabiliser or rheology modifier. 43,44 Odd numbered fatty acids are widespread in fats from the animal kingdom, but are rare in plants, 45 suggesting that small amounts of brute fats are nowadays in WNRS. Every bit for castor wax, animal fatty might have been added to the paint conception, but could likewise be a residue of the paint preparation process.
A newly developed GC-MS analytical process 22 was adopted, to both qualitatively and quantitatively determine free fatty and dicarboxylic acids in the model paint samples, likewise every bit free carboxylates of fatty and dicarboxylic acids (that are not bound to the polymeric network, nor to glycerides). This process entails two subsequent derivatisations on the same sample, first with HMDS, which is able to derivatise only free fatty and free dicarboxylic acids, and the second with BSTFA, which also derivatises the metallic soaps of complimentary fatty and dicarboxylic acids. Fig. 4–6 show the chromatograms of: (i) FFA – the fractions relative to the free fatty and dicarboxylic acids, and (2) FFA + MS: the fraction relative to free fatty acids and dicarboxylic acids plus costless carboxylates of fatty and dicarboxylic acids (MS).
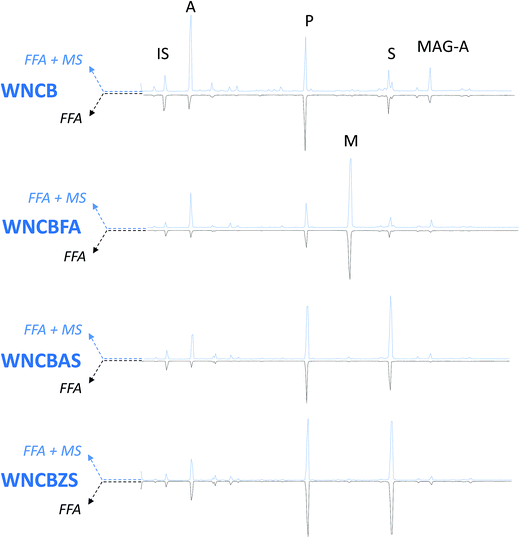 |
| | Fig. four GC-MS chromatograms of WNCB model paints. IS: internal standard; A: azelaic acid; P: palmitic acid; M: margaric acid; Southward: stearic acid; Magazine-A: azelaic acrid monoacylglycerol. Blue traces – pointing up-chromatograms relative to costless fat and dicarboxylic acids + carboxylates of fatty and dicarboxylic acids (FFA + MS); black traces – pointing down – chromatograms relative to gratis fatty and dicarboxylic acids (FFA). All acids are separated in the grade of silylesters. | |
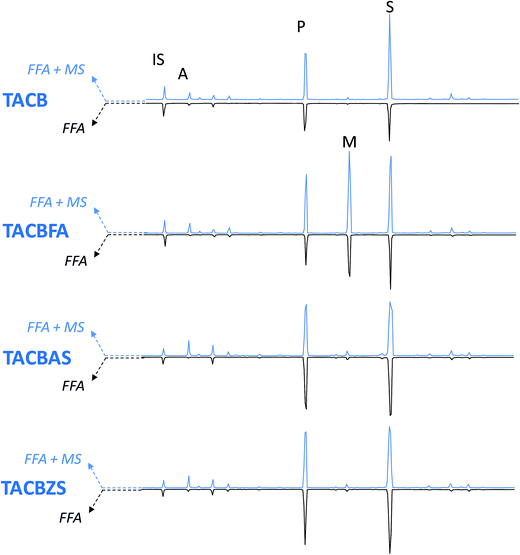 |
| | Fig. five GC-MS chromatograms of TACB model paints. IS: internal standard; A: azelaic acid; P: palmitic acid; M: margaric acrid; South: stearic acid. Blue traces – pointing up-chromatograms relative to free fatty and dicarboxylic acids + + carboxylates of fatty and dicarboxylic acids (FFA + MS); black traces – pointing down – chromatograms relative to complimentary fatty and dicarboxylic acids (FFA). All acids are separated in the course of silylesters. | |
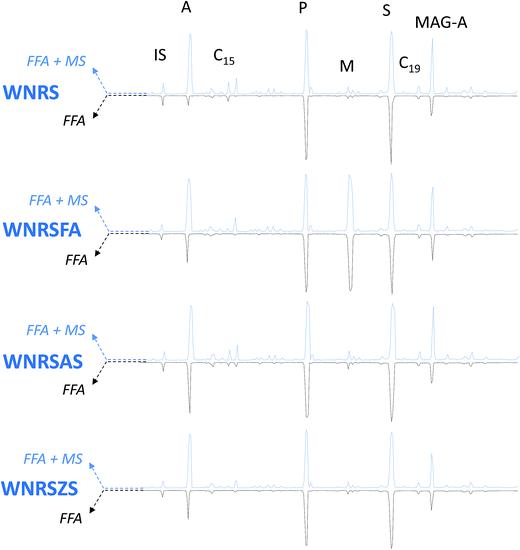 |
| | Fig. 6 GC-MS chromatograms of WNRS model paints. IS: internal standard; A: azelaic acrid; C 15 : pentadecanoic acrid; P: palmitic acid; M: margaric acid; Southward: stearic acid; C 19 : nonadecanoic acid; Magazine-A: azelaic acid monoacylglycerol. Blueish traces – pointing upwardly-chromatograms relative to gratis fatty and dicarboxylic acids + + carboxylates of fatty and dicarboxylic acids (FFA + MS); blackness traces – pointing downwardly – chromatograms relative to free fat and dicarboxylic acids (FFA). All acids are separated in the form of silylesters. | |
Table 4 summarises the results of the quantitative analyses performed on the GC-MS information. Values reported are the boilerplate of measurements carried out on triplicate samples. Confidence intervals reported are those relative to a conviction level of 95%. Information reported in Table 4 were used to build the histograms reported in Fig. 7.
Table 4 Results of the quantitative analyses performed on the GC-MS data. A/P: ratio between the relative content of azelaic acid and palmitic acid; P/S: ratio betwixt the relative content of palmitic acid and stearic acid; ∑dicarboxylic acids: sum of the relative content of dicarboxylic acids (azelaic, suberic and sebacic acids); weight%: measure of the degree of hydrolysis and caste of saponification – more details are reported in the text
| Sample | Fraction | A/P | ∑dicarboxylic acids (weight%) | P/South | weight% |
| WNCB | FFA | 0.3 ± 0.1 | 0.06% ± 0.01% | 1.5 ± 0.2 | 0.3% ± 0.0% |
| FFA + MS | 1.2 ± 0.ane | 0.xiv% ± 0.03% | 2.four ± 0.two | 0.five% ± 0.1% |
| WNCBFA | FFA | 0.3 ± 0.i | 0.06% ± 0.01% | 2.2 ± 0.3 | 0.3% ± 0.1% |
| FFA + MS | 0.9 ± 0.1 | 0.13% ± 0.03% | 2.vi ± 0.seven | 0.8% ± 0.4% |
| WNCBAS | FFA | 0.2 ± 0.0 | 0.05% ± 0.01% | 0.vii ± 0.ane | 0.6% ± 0.1% |
| FFA + MS | 0.3 ± 0.0 | 0.07% ± 0.00% | 0.7 ± 0.1 | ane.3% ± 0.4% |
| WNCBZS | FFA | 0.i ± 0.0 | 0.05% ± 0.01% | 0.half-dozen ± 0.0 | 1.0% ± 0.3% |
| FFA + MS | 0.2 ± 0.0 | 0.09% ± 0.02% | 0.7 ± 0.0 | 1.9% ± 0.6% |
| TACB | FFA | 0.ane ± 0.0 | 0.02% ± 0.01% | 0.half-dozen ± 0.0 | 0.5% ± 0.3% |
| FFA + MS | 0.one ± 0.0 | 0.00% ± 0.00% | 0.5 ± 0.0 | 1.iv% ± 0.3% |
| TACBFA | FFA | 0.0 ± 0.0 | 0.01% ± 0.00% | 0.v ± 0.0 | 0.six% ± 0.ii% |
| FFA + MS | 0.1 ± 0.0 | 0.04% ± 0.01% | 0.5 ± 0.0 | 2.0% ± 0.viii% |
| TACBAS | FFA | 0.0 ± 0.0 | 0.01% ± 0.01% | 0.6 ± 0.0 | one.vii% ± 0.one% |
| FFA + MS | 0.1 ± 0.0 | 0.06% ± 0.03% | 0.6 ± 0.1 | ii.vii% ± 0.2% |
| TACBZS | FFA | 0.0 ± 0.0 | 0.02% ± 0.01% | 0.6 ± 0.0 | 1.4% ± 0.ii% |
| FFA + MS | 0.1 ± 0.0 | 0.02% ± 0.00% | 0.6 ± 0.one | 2.8% ± 0.viii% |
| WNRS | FFA | 0.v ± 0.2 | 0.21% ± 0.14% | 0.8 ± 0.0 | one.9% ± 0.5% |
| FFA + MS | one.two ± 0.1 | ane.03% ± 0.31% | 0.8 ± 0.1 | 3.4% ± 0.5% |
| WNRSFA | FFA | 0.five ± 0.ane | 0.34% ± 0.06% | 0.eight ± 0.0 | 2.three% ± 0.7% |
| FFA + MS | i.1 ± 0.0 | 0.67% ± 0.34% | 0.ix ± 0.one | 4.ane% ± 0.4% |
| WNRSAS | FFA | 0.2 ± 0.ane | 0.23% ± 0.08% | 0.7 ± 0.1 | 2.2% ± 0.2% |
| FFA + MS | 0.nine ± 0.2 | 1.05% ± 0.23% | 0.vii ± 0.0 | 4.7% ± 0.iii% |
| WNRSZS | FFA | 0.three ± 0.one | 0.33% ± 0.10% | 0.seven ± 0.0 | 2.half dozen% ± 0.4% |
| FFA + MS | i.0 ± 0.0 | 0.85% ± 0.37% | 0.7 ± 0.one | 6.0% ± 1.2% |
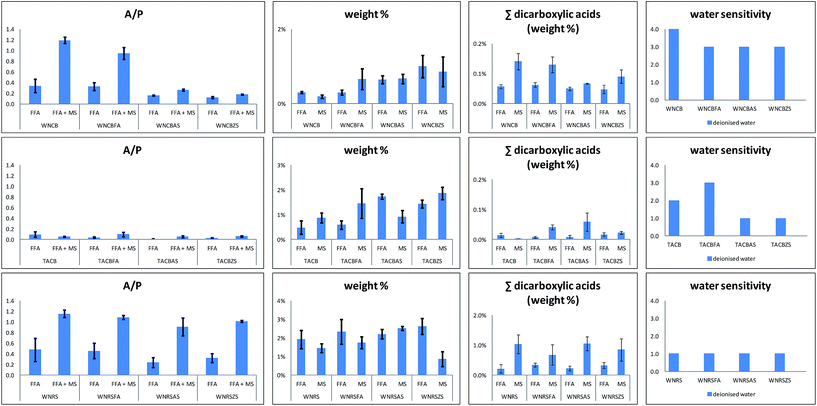 |
| | Fig. seven A/P: ratios betwixt the relative content of azelaic acrid and that of palmitic acrid: weight%: FA – measure out of the degree of hydrolysis, MS – measure of the degree of formation of metal soaps; ∑dicarboxylic acids (weight%): FFA relative content of gratuitous dicarboxylic acids. ∑dicarboxylic acids (weight%): MS – relative content of metal soaps of dicarboxylic acids; water sensitivity. Analyses were performed in triplicates and confidence intervals were calculated at a 95% confidence level. | |
The P/S values of the unmodified TACB and WNRS paints were noted as being quite low, particularly considering the type of oils used. 36 This ratio, together with the presence of Zn (in TACB) and Al and Zn (in WNRS), strongly suggest that the paints contained Zn and Al stearates, which were likely to have been added by the paint manufacturers. Fig. vii depicts the ratios between the relative content of azelaic acid and that of palmitic acrid (A/P) obtained from the FFA and FFA + MS fractions.
Dicarboxylic acids are the final product of oxidation of drying oils, formed as a natural outcome of auto-oxidative reactions taking place during curing. Assuming that saturated monocarboxylic acids are stable over time, 46 the A/P ratio classically calculated by GC/MS is related to the caste of oxidation of a pigment. 20 Costless dicarboxylic and monocarboxylic acids in a paint are the issue of the hydrolysis of triglycerides. Keeping this in listen, the A/P values in the FFA and FFA + MS fractions relate to the caste of hydrolysis of azelaic acid, and of the formation of its corresponding metallic lather with respect to palmitic acid. 22 In the TACB paint, the A/P ratios are never above 0.i. The WNRS paints were characterized by a high degree of formation of metal soaps containing azelaic acid with respect to palmitic acid – the A/P ratio values of the FFA + MS fractions were always significantly college than those of the FFA fraction. In the WNCB paints, although the A/P ratio values of the FFA + MS fractions were always higher than those of the FFA fractions, the samples with added stearates comprise significantly lower A/P values than the others.
In addition to the peaks ascribable to azelaic (A), palmitic (P), margaric (Yard) and stearic (S) acids, the chromatograms of the WNRS samples also testify the presence of odd numbered fat acids (pentadecanoic, margaric, and nonadecanoic acids – present in all chromatograms), confirming the observations of HPLC-MS analyses suggesting the presence of a fat of animal origin 45 in this pigment. The chromatograms of all Due west&N paints nowadays a pinnacle ascribable to 9-(2,3-dihydroxypropoxy)-nine-oxononanoic acid (azelaic acid monoacylglycerol, Magazine-A). The relative intensity of this height is (in the majority of the samples) higher in the fraction derivatised with BSTFA than in the fraction derivatised with HMDS, suggesting that the gratuitous moiety of azelaic acid in the monoacylglycerol forms metallic soaps. The presence of consequent amounts of metallic soaps of azelaic acrid monoacylglycerol leads to the supposition that azelaic acid, bound from 1 side to glycerol, is likely to be nowadays as carboxylate also in diglycerides and triglycerides, which are not detected using gas chromatography due to their depression volatility. This supports the recently presented model of an oil paint layer, co-ordinate to which a significant fraction of carboxylate groups vest to the covalent role of the oil network, leading to the germination of a ionomer-like network. 28
Fig. 7 as well shows a measure of the caste of hydrolysis and the caste of germination of metal soaps of the model paint samples. The measure of the degree of hydrolysis of the model paint samples (FA weight% in Fig. 7) was calculated as the sum of the weight content of lauric (L), suberic (Sub), azelaic (A), myristic (Yard), sebacic (Seb), palmitic (P), oleic (O) and stearic (Southward) acids measured in the FFA fraction, normalised to the sample weight.
Mensurate of the caste of hydrolysis:
The measure out of the degree of germination of soaps of fatty and dicarboxylic acids of the model pigment samples (MS weight% in Fig. 7) was calculated as the difference between the sum of the weight content of lauric, suberic, azelaic, myristic, sebacic, palmitic, oleic and stearic acids measured in the FFA + MS fraction, and the sum of the weight content of lauric, suberic, azelaic, myristic, sebacic, palmitic, oleic and stearic acids measured in the FFA fraction, also normalised to the sample weight.
Mensurate of formation of soaps of fatty and dicarboxylic acids:
The adamant amounts refer only to free fatty and dicarboxylic acids and their relative metal soaps, and thus not to those acids which are still jump to glycerides, nor to those that are incorporated into the polymeric/ionomer-like network. Also, the data refer to the sample weight – which accounts for the binder, pigment and whatever other condiment present – and non to the organic content only, which is not known. Every bit a event, the data from different paints cannot be compared quantitatively, although this can exist done within the paints with the same pigment and inside one brand.
The addition of complimentary fatty acids (in this instance margaric acid, which was not included in the calculations) causes an intrinsic increment of the free acidic moieties nowadays in the paint, but does not announced to catalyse the hydrolysis of the paint (FFA weight% in Fig. 7 and Tabular array 3 relative to samples WNCB/WNCBFA, TACB/TACBFA, and WNRS/WNRSFA). In samples WNCBFA and TACBFA, the added gratis fat acids promptly formed metallic soaps (see Fig. four–half-dozen). Indicating the average value of the ratio betwixt the amount of margaric acid in the FFA + MS fraction and that of the respective FFA fraction with MFFA + MS/MFFA, we obtained: MFFA + MS/MFFA(WNCB) = 2.1; MFFA + MS/MFFA(TACB) = 2.7; MFFA + MS/MFFA(WNRS) = 0.9 (Fig. 4–6 chromatograms relative to WNCBFA, TACBFA and WNRSFA).
Both Al and Zn stearates do announced to increment the relative content of gratuitous fatty acids in the paints, especially in the cobalt blue paints (see FFA weight% in Fig. 7 relative to samples WNCB/WBCBAS/WNCBZS, TACB/TACBAS/TACBZS, and WNRS/WNRSAS/WNRSZS). This could be due to the fact that technical stearates comprise complimentary fatty acids. 11,12 . Moreover it has been suggested that metal soaps might catalyse the hydrolysis of triglycerides. 47–49
The relative content of free dicarboxylic acids (FFA∑ dicarboxylic acids (weight%) in Fig. 7) with respect to the sample weight was also calculated equally the sum of the weight content of suberic, azelaic and sebacic acids measured in the FFA fraction, normalised to the sample weight.
Relative content of free dicarboxylic acids:
Differences in the content of free dicarboxylic acids due north paints of the same series (aforementioned brand and some pigment) practice non appear significant: the amount of dicarboxylic acids – which are partially h2o soluble does not appear to relate to water sensitivity, as the more water sensitive samples do non incorporate significantly higher amounts of complimentary dicarboxylic acids than the less water sensitive samples. Previous enquiry showed that irrespective of the overall caste of oxidation of the pigment, ethanol extracted a relatively college corporeality of gratuitous dicarboxylic acids from water-sensitive with respect to non-h2o sensitive paints. 19 Nosotros can thus hypothesise that water sensitive samples are more attainable to water ingress, either by swelling the surface or capillary penetration, resulting in a more effective solubilisation of dicarboxylic acids. The condition of the paint may be explained past an insufficient degree of crosslinking of the dry film, making the pigment layers less tightly bound, and thus susceptible to swelling by polar solvents. Conversely, we can hypothesise that not-water sensitive paints are characterised by a more extended polymeric network, and are thus not penetrated by water, resulting in a express access of water to any free dicarboxylic acids present in the paint layers. As a result, the relatively loftier content of dicarboxylic acids in the extracts of water sensitive oil paint films may non be a directly cause of water sensitivity, but may be a consequence/symptom of the lack of formation of a well-developed polymeric/ionomeric paint system.
The relative content of metal soaps of dicarboxylic acids (MS ∑dicarboxylic acids (weight%) in Fig. seven) with respect to the sample weight was likewise calculated equally the sum of the difference between the sum of the weight content of suberic, azelaic and sebacic acids measured in the FFA + MS fraction, and the sum of weight content of suberic, azelaic and sebacic acids measured in the FFA S fraction, normalised to the sample weight.
Relative content of metallic soaps of dicarboxylic acids:
It was proposed that metallic soaps of azelaic and other diacids class a relatively stable metallic coordinated tri-dimensional network because of their chain building ability, 21 contributing to paint stability. These data indicate that water sensitivity does not correlate with the relative amount of gratis metal soaps of dicarboxylic acids, as, within the same ready of paints, the more than water sensitive samples do not take lower proportions of gratis metallic soaps of dicarboxylic acids.
An important observation noted from these data is that the relative content of free fatty acids and complimentary metallic soaps was below 10% past weight in all of the samples investigated. This amount is not sufficient to justify the intensity of the distinctive FTIR absorption band at 1711 cm −1 ascribable to costless fatty acids (Fig. 2) in the WNRS sample, nor those of the metal soaps (absorption band at around 1590 cm −1 ascribable to metal carboxylates) in the samples WNCB and TACB (Fig. ane and 2). To examine this further, fresh paints were prepared using haematite in linseed oil, and divided into two aliquots, stearic acid (10% w/west) was added to one aliquot and Zn stearate (10% w/west) was added to the other. Both were analysed past FTIR (IR spectra are reported in ESI); The IR spectra clearly show that the intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration relative to free fat acids (1710 cm −i ) and that of the C
O stretching vibration relative to free fat acids (1710 cm −i ) and that of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration relative to Zn stearate (1538 cm −1 ) is significantly lower than the intensity of the C
O stretching vibration relative to Zn stearate (1538 cm −1 ) is significantly lower than the intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration relative to the oil glyceride (1743 cm −1 )southward. In addition, the IR absorptions ascribable to metal carboxylates in samples WNCB and TACB fall within the wavelength ranges assigned to amorphous metal carboxylates, 28,30 which are not analysed by the GC-MS procedure, able only to discover carboxylates of fat and dicarboxylic acids that are not spring to the polymeric/ionomeric network. The crosslinked network derives from the polymerization of polyunsaturated fat acids. A polyunsaturated fat acid, begetting more than one unsaturation, may crosslink at one carbon and oxidise at another carbon, leading to the formation of dicarboxylic acids, which are linked via a C–C or C–O–C bonds to other acids in the network. When combined, these observations lead us to conclude that the band at 1711 cm −1 in the WNRS sample, and the bands effectually 1590 cm −1 in the samples WNCB and TACB must be ascribed to acidic moieties and carboxylate groups, the majority of which are fastened to the polymeric/ionomeric network, in which acrid and metal coordinated carboxylate moieties coexist in a complex structure, which represents the main organic constituent of a mature paint film.
O stretching vibration relative to the oil glyceride (1743 cm −1 )southward. In addition, the IR absorptions ascribable to metal carboxylates in samples WNCB and TACB fall within the wavelength ranges assigned to amorphous metal carboxylates, 28,30 which are not analysed by the GC-MS procedure, able only to discover carboxylates of fat and dicarboxylic acids that are not spring to the polymeric/ionomeric network. The crosslinked network derives from the polymerization of polyunsaturated fat acids. A polyunsaturated fat acid, begetting more than one unsaturation, may crosslink at one carbon and oxidise at another carbon, leading to the formation of dicarboxylic acids, which are linked via a C–C or C–O–C bonds to other acids in the network. When combined, these observations lead us to conclude that the band at 1711 cm −1 in the WNRS sample, and the bands effectually 1590 cm −1 in the samples WNCB and TACB must be ascribed to acidic moieties and carboxylate groups, the majority of which are fastened to the polymeric/ionomeric network, in which acrid and metal coordinated carboxylate moieties coexist in a complex structure, which represents the main organic constituent of a mature paint film.
4. Conclusions
Gratuitous fatty and dicarboxylic acids, and carboxylates of fatty and dicarboxylic acids were determined qualitatively and quantitatively. The adamant species stand for the non-bonded fraction of a mature oil paint film. This information was interpreted in relation to the water sensitivity of the paints. Data clearly show that paints with water sensitivity rating from one to 4 (from not-water sensitive to highly water sensitive) are characterised by relatively similar amounts of costless and dicarboxylic acids, and therefore no meaning trend was observed for these parameters.
Comparison of spectroscopic information with the results of the GC-MS quantification of free fatty acids, costless dicarboxylic acids, and their relative metal soaps indicated that most of the metal carboxylates and acidic moieties of free carboxylic acids in these paints are not part of the non-bonded fraction, but are associated with the polymeric/ionomeric network. In understanding with recent findings, 21,28,30,l we support the hypothesis of a model of polymeric/ionomeric network of a mature paint motion-picture show composed of crosslinked and partially hydrolysed glycerides, a substantial portion of which are metallic coordinated. The results of this report propose that the nature of the polymeric/ionomeric network is a significant determining factor impacting on the development of water sensitivity. It has been previously shown that water sensitivity is dependent primarily on the pigment blazon. 14 In this report we demonstrated that additives may too influence the molecular composition of a paint film (as observed from the analysis of molecular profiles of free carboxylic acids and their relative metal soaps), and may too affect water sensitivity: added Zn or Al stearates generally cause a pocket-sized decrease of h2o sensitivity, while added free fat acids do not evidence a consistent trend. Hence it is likely that non only pigments, but also additives tin can affect the curing process of the paint, influencing, together with external factors, 51 ** the caste of oxidation/crosslinking, and thus the nature of the mature polymeric/ionomeric network, which in turn contributes to the formation of water sensitive or h2o resistant paint films. Research is still necessary to investigate this complex system further, requiring the development of new belittling approaches, which can besides investigate the molecular and concrete limerick of the polymeric/ionomeric network.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was performed inside the context of the JPI CMOP projection: "Cleaning Modernistic Oil Paints" (Heritage Plus Joint Call projection 2015–2018). http://www.tate.org.britain/almost-u.s./projects/cleaning-modern-oil-paints-0 Silvia Pizzimenti is kindly acknowledged for her help with FTIR measurements. References
- F. C. Izzo, G. J. van den Berg, H. van Keulen, B. Ferriani and E. Zendri, in Issues in gimmicky oil paint, Springer, 2014, pp. 75–104 Search PubMed.
- T. Learner, in Conservation of easel paintings, ed. J. H. Stoner and R. Rushfield, Routledge, Abingdon, Oxon/New York, 2013 Search PubMed.
- A. Burnstock and K. J. van den Berg, in Problems in Contemporary Oil Pigment, Springer, 2014, pp. 1–19 Search PubMed.
- A. Burnstock, K. J. van den Berg, F. van Gurp, Southward. Bayliss and K. Ovink, in Sources in Fine art Technology, Dorsum to Basics, ed. S. E.-Green, J. H. Townsend, K. Pilz, South. Kroustallis and I. v. Leeuwen, Archetype, 2016, pp. 43–50 Search PubMed.
- Thou. Clarke, Stud. Conserv., 2009, 54, 160–169 CrossRef.
- 1000. A. Smith, A single-shot separation and identification technique for water extractable additives in acrylic emulsion paints, 2005 Search PubMed.
- M. F. Silva, One thousand. T. Doménech-Carbó and L. Osete-Cortina, J. Anal. Appl. Pyrolysis, 2015, 113, 606–620 CrossRef CAS.
- S. Zumbül, F. Attanasio, N. C. Scherrer, West. Müller, N. Fenners and W. Caseri, in Mod Paints Uncovered: Proceedings from the Tate Modern Symposium, ed. T. Learner, P. Smithen, J. Krueger, and Thousand. Schilling, The Getty Conservation Institute, Los Angeles, 2008, pp. 257–268 Search PubMed.
- A. Burnstock, G. J. Van den Berg, S. de Groot and L. Wijnberg, in Modernistic paints uncovered: proceedings from the Modern Paints Uncovered Symposium, ed. T. Learner, P. Smithen, J. Krueger and Chiliad. Schilling, The Getty Conservation Institute, Los Angeles, 2008, pp. 177–188 Search PubMed.
- R. Mayer, Artist'south Handbook of Materials and Techniques: Revised and Updated, Viking Books, 1991 Search PubMed.
- L. Mills, A. Burnstock, F. Duarte, South. de Groot, L. Megens, G. Bisschoff, H. van Keulen, and K. J. van den Berg, in ICOM Committee for Conservation 15th Triennial Coming together, New Delhi, 2008, pp. 651–659 Search PubMed.
- C. S. Tumosa, Waac newsletter, 2001, vol. 23 Search PubMed.
- 1000. Silvester, A. Burnstock, Fifty. Megens, T. Learner, G. Chiari and K. J. van den Berg, Stud. Conserv., 2014, 59, 38–51 CrossRef CAS.
- A. Burnstock, J. Lee, Yard. J. van den Berg and B. Ormsby, in Dall'olio all'acrilico, dall'impressionismo all'arte contemporanea: Studi, ricerche, indagini scientifiche ed interventi conservative: proceedings from the VII International Congress on Colour and Conservation, Cesmar7 and Il Prato publishing house srl, Milano, 2015, vol. 2016, pp. 66–76 Search PubMed.
- G. Silvester, An experimental investigation of the germination of sulphates in oil paint films exposed to gaseous sulphur dioxide with item reference to the relationship betwixt these sulphates and water sensitivity Department of Conservation & Applied science, Courtauld Establish of Fine art, 2011 Search PubMed.
- H. Storm, A. Burnstock, P. Saltmarsh and K. van den Berg, in New Insights into the Cleaning of Paintings Proceedings from the Cleaning 2010 International Conference Universidad Politécnica de Valencia and Museum Conservation Institute, ed. M. F. Mecklenburg, A. East. Charola and R. J. Koestler, 2013, pp. 107–114 Search PubMed.
- A. Cooper, A. Burnstock, One thousand. J. van den Berg and B. Ormsby, in Issues in contemporary oil paint, Springer, 2014, pp. 295–310 Search PubMed.
- L. Mills, Department of Conservation & Technology, Courtauld Institute of Art, 2008 Search PubMed.
- J. Lee, I. Bonaduce, F. Modugno, J. La Nasa, B. Ormsby and Thousand. J. van den Berg, Microchem. J. Search PubMed , accepted for publication.
- J. Mills and R. White, Organic chemistry of museum objects, Routledge, 2012 Search PubMed.
- J. J. Boon, F. Hoogland and K. Keune, in AIC Paintings Specialty Grouping Postprints, Providence, American Institute for Conservation of Celebrated & Artistic Works, Rhode Island, 2006, vol. xix, pp. sixteen–23 Search PubMed.
- I. Degano and J. La Nasa, Top. Curr. Chem., 2016, 374, 20 CrossRef PubMed.
- D. Banti, Department of Conservation & Technology, Courtauld Institute of Art, 2017 Search PubMed.
- C. E. Dillon, A. F. Lagalante and R. C. Wolbers, Stud. Conserv., 2014, 59, 52–62 CrossRef CAS.
- R. Mazzeo, Southward. Prati, M. Quaranta, E. Joseph, E. Kendix and Chiliad. Galeotti, Anal. Bioanal. Chem., 2008, 392, 65–76 CrossRef CAS PubMed.
- One thousand. F. Mecklenburg, C. S. Tumosa and D. Erhardt, MRS Online Proc. Libr., 2004, 852, OO3.1 Search PubMed.
- L. Bay, A. Burnstock, J. Lee, B. Ormsby, and K. J. van den Berg, Water sensitivity of modern oil paintings. In ICOM-CC 18th Triennial Conference Preprints, Copenhagen, ed. J. Bridgland, Paris, International Quango of Museums, 4–8 September 2017, fine art. 1302 Search PubMed.
- J. J. Hermans, K. Keune, A. van Loon, R. W. Corkery and P. D. Iedema, RSC Adv., 2016, half dozen, 93363–93369 RSC.
- 50. Monico, K. Janssens, 1000. Cotte, L. Sorace, F. Vanmeert, B. Chiliad. Brunetti and C. Miliani, Microchem. J., 2016, 124, 272–282 CrossRef CAS.
- J. J. Hermans, K. Keune, A. van Loon and P. D. Iedema, J. Anal. At. Spectrom., 2015, 30, 1600–1608 RSC.
- V. Otero, D. Sanches, C. Montagner, M. Vilarigues, 50. Carlyle, J. A. Lopes and M. J. Melo, J. Raman Spectrosc., 2014, 45, 1197–1206 CrossRef CAS.
- S. Mukherjee and A. Datta, J. Nanosci. Nanotechnol., 2009, 9, 5237–5242 CrossRef CAS PubMed.
- K. Helwig, Due east. A. Moffatt, M.-C. Corbeil and D. Duguay, Periodical of the Canadian Association for Conservation (CAC), 2015, twoscore, 19–34 Search PubMed.
- L. Monico, F. Rosi, C. Miliani, A. Daveri and B. Thousand. Brunetti, Spectrochim. Acta, Part A, 2013, 116, 270–280 CrossRef CAS PubMed.
- H. Edwards and P. Hardman, J. Mol. Struct., 1992, 273, 73–84 CrossRef CAS.
- I. Degano, J. La Nasa, E. Ghelardi, F. Modugno and Chiliad. P. Colombini, Talanta, 2016, 161, 62–lxx CrossRef CAS PubMed.
- J. La Nasa, 1000. Zanaboni, D. Uldanck, I. Degano, F. Modugno, H. Kutzke, Eastward. S. Tveit, B. Topalova-Casadiego and G. P. Colombini, Anal. Chim. Acta, 2015, 896, 177–189 CrossRef CAS PubMed.
- J. La Nasa, I. Degano, F. Modugno and M. P. Colombini, Anal. Chim. Acta, 2013, 797, 64–eighty CrossRef CAS PubMed.
- J. La Nasa, E. Ghelardi, I. Degano, F. Modugno and M. P. Colombini, J. Chromatogr. A, 2013, 1308, 114–124 CrossRef CAS PubMed.
- J. La Nasa, I. Degano, F. Modugno and One thousand. P. Colombini, Polym. Degrad. Stab., 2014, 105, 257–264 CrossRef CAS.
- J. La Nasa, I. Degano, L. Brandolini, F. Modugno and I. Bonaduce, Anal. Chim. Acta, 2018 DOI:x.1016/j.aca.2017.12.047.
- H. Tempest, A. Burnstock, P. Saltmarsh and K. Van den Berg, Sensitivity of oil paint surfaces to aqueous and other solvents, New insights into the cleaning of paintings, Proceedings from the cleaning 2010 International Conference, Valencia, 2010 Search PubMed.
- R. Ploeger and O. Chiantore, 2013.
- Southward. Zumbuhl, Due east. S. Ferreira, N. C. Scherrer and V. Schaible, in New Insights into the Cleaning of Paintings Proceedings from the Cleaning 2010 International Conference Universidad Politécnica de Valencia and Museum Conservation Institute, ed. 1000. F. Mecklenburg, A. E. Charola and R. J. Koestler; 2013, pp. 97–105 Search PubMed.
- Thou. Diedrich and G. P. Henschel, Mol. Nutr. Food Res., 1990, 34, 935–943 CAS.
- I. Bonaduce, L. Carlyle, 1000. P. Colombini, C. Duce, C. Ferrari, E. Ribechini, P. Selleri and K. R. Tine, PLoS One, 2012, vii, e49333 CAS.
- J. La Nasa, F. Modugno, M. Aloisi, A. Lluveras Tenorio and I. Bonaduce, Anal. Chim. Acta, 2018, 1001, 51–58 CrossRef CAS PubMed.
- M. Cotte, E. Checroun, J. Susini, P. Dumas, P. Tchoreloff, M. Besnard and P. Walter, Talanta, 2006, 70, 1136–1142 CrossRef CAS PubMed.
- Thou. Cotte, E. Checroun, Westward. De Nolf, Y. Taniguchi, Fifty. De Viguerie, Yard. Burghammer, P. Walter, C. Rivard, M. Salomé, K. Janssens and J. Susini, Stud. Conserv., 2017, 62, 2–23 CrossRef CAS.
- J. A. Lee, B. A. Ormsby, A. Burnstock, M. Schilling, and K. J. van den Berg, The chemic characterisation of water-sensitive modernistic oil pigment swatches by Winsor & Newton, in ICOM-CC 18th Triennial Conference Preprints, Copenhagen, ed. J. Bridgland, International Council of Museums, Paris, 4–viii September 2017, fine art. 1604 Search PubMed.
- Yard. Keune, F. Hoogland, J. Benefaction, D. Peggie and C. Higgitt, in ICOM Committee for Conservation 15th Triennial Meeting, New Delhi, 2008, pp. 833–842 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra13364b |
| ‡ Tate, Modern Oil Research Consortium, Available at: http://world wide web.tate.org.uk/about/projects/modernistic-oils-researchconsortium |
§ Artificial ageing was done at Stichting Restauratie Atelier Limburg (SRAL). Illumination was provided past 36 W Philips color 96.5 fluorescent lamps, with UV filtering (transmission 15 watt per lumen), rendering a measured output of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lux at the sample surface. Temperature was 25 °C and RH threescore% on average and the total ageing time of 1390 hours. The total ageing time of 1390 hours is calculated to be equivalent to 20-four years of exhibition in recommended museum weather, 200 lux, eight hours a day, assuming reciprocity. 11,20 000 lux at the sample surface. Temperature was 25 °C and RH threescore% on average and the total ageing time of 1390 hours. The total ageing time of 1390 hours is calculated to be equivalent to 20-four years of exhibition in recommended museum weather, 200 lux, eight hours a day, assuming reciprocity. 11,20 |
| ¶ For Winsor and Newton information were taken directly from the tubes. For Talens the data was available at: https://www.royaltalens.com/media/1412025/Consumentenfolder_olieverf_EN.pdf [accessed 15/12/2016]. Full formula and other information on the pigments tin can be establish in the Colour of Art Paint Database; http://www.artiscreation.com/[accessed 15/12/2016]. |
| || J. La Nasa, A. Lluveras-Tenorio, F. Modugno, I. Bonaduce, "2-steps analytical approach for the characterization and quantification of metal soaps and resinates in paint samples", in preparation. |
| ** F. Modugno, F. di Gianvincenzo, I. Degano, I. Bonaduce, K. J. van den Berg, "On the influence of relative humidity on the oxidation and hydrolysis of fresh and naturally aged oil paints ", 2018, in preparation. |
|
| This periodical is © The Royal Society of Chemistry 2018 |



![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration related to the formation of free fat acids as a result of the hydrolysis of triglycerides, and, to a sure extent, oxidation with germination of dicarboxylic acids. This is in understanding with the results obtained for other paint reconstructions consisting of linseed oil and Fe based pigments (reddish ochre, Prussian blue and red bole) 25 confirming the observation that Fe-based pigments promote the hydrolysis of triglycerides. 26 Complimentary fatty acids could not exist distinguished in WNCB and TACB (Fig. 1 and 2), however this has been observed in other paints containing Co and Zn containing-pigments (zinc white and cobalt light-green). 14,25,27 A separate carbonyl band with absorptions at ca.1740 cm −1 and ca. 1710 cm −1 has been associated with h2o sensitive oil paints in a related study. 14,27
O stretching vibration related to the formation of free fat acids as a result of the hydrolysis of triglycerides, and, to a sure extent, oxidation with germination of dicarboxylic acids. This is in understanding with the results obtained for other paint reconstructions consisting of linseed oil and Fe based pigments (reddish ochre, Prussian blue and red bole) 25 confirming the observation that Fe-based pigments promote the hydrolysis of triglycerides. 26 Complimentary fatty acids could not exist distinguished in WNCB and TACB (Fig. 1 and 2), however this has been observed in other paints containing Co and Zn containing-pigments (zinc white and cobalt light-green). 14,25,27 A separate carbonyl band with absorptions at ca.1740 cm −1 and ca. 1710 cm −1 has been associated with h2o sensitive oil paints in a related study. 14,27 



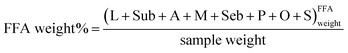

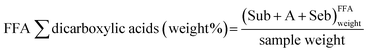
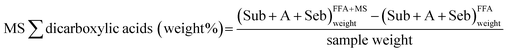
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration relative to free fat acids (1710 cm −i ) and that of the C
O stretching vibration relative to free fat acids (1710 cm −i ) and that of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration relative to Zn stearate (1538 cm −1 ) is significantly lower than the intensity of the C
O stretching vibration relative to Zn stearate (1538 cm −1 ) is significantly lower than the intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration relative to the oil glyceride (1743 cm −1 )southward. In addition, the IR absorptions ascribable to metal carboxylates in samples WNCB and TACB fall within the wavelength ranges assigned to amorphous metal carboxylates, 28,30 which are not analysed by the GC-MS procedure, able only to discover carboxylates of fat and dicarboxylic acids that are not spring to the polymeric/ionomeric network. The crosslinked network derives from the polymerization of polyunsaturated fat acids. A polyunsaturated fat acid, begetting more than one unsaturation, may crosslink at one carbon and oxidise at another carbon, leading to the formation of dicarboxylic acids, which are linked via a C–C or C–O–C bonds to other acids in the network. When combined, these observations lead us to conclude that the band at 1711 cm −1 in the WNRS sample, and the bands effectually 1590 cm −1 in the samples WNCB and TACB must be ascribed to acidic moieties and carboxylate groups, the majority of which are fastened to the polymeric/ionomeric network, in which acrid and metal coordinated carboxylate moieties coexist in a complex structure, which represents the main organic constituent of a mature paint film.
O stretching vibration relative to the oil glyceride (1743 cm −1 )southward. In addition, the IR absorptions ascribable to metal carboxylates in samples WNCB and TACB fall within the wavelength ranges assigned to amorphous metal carboxylates, 28,30 which are not analysed by the GC-MS procedure, able only to discover carboxylates of fat and dicarboxylic acids that are not spring to the polymeric/ionomeric network. The crosslinked network derives from the polymerization of polyunsaturated fat acids. A polyunsaturated fat acid, begetting more than one unsaturation, may crosslink at one carbon and oxidise at another carbon, leading to the formation of dicarboxylic acids, which are linked via a C–C or C–O–C bonds to other acids in the network. When combined, these observations lead us to conclude that the band at 1711 cm −1 in the WNRS sample, and the bands effectually 1590 cm −1 in the samples WNCB and TACB must be ascribed to acidic moieties and carboxylate groups, the majority of which are fastened to the polymeric/ionomeric network, in which acrid and metal coordinated carboxylate moieties coexist in a complex structure, which represents the main organic constituent of a mature paint film.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lux at the sample surface. Temperature was 25 °C and RH threescore% on average and the total ageing time of 1390 hours. The total ageing time of 1390 hours is calculated to be equivalent to 20-four years of exhibition in recommended museum weather, 200 lux, eight hours a day, assuming reciprocity. 11,20
000 lux at the sample surface. Temperature was 25 °C and RH threescore% on average and the total ageing time of 1390 hours. The total ageing time of 1390 hours is calculated to be equivalent to 20-four years of exhibition in recommended museum weather, 200 lux, eight hours a day, assuming reciprocity. 11,20 
0 Response to "How To Get A Chemical Reaction With Oil Paint"
Post a Comment